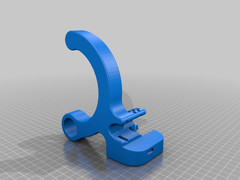
This device was designed to allow you to use your forearm/elbow to open doors with normal, lever-shaped handles that are vertically operated with a downward movement, similar to the one displayed in the image below:
Opening doors with the elbow/forearm can significantly reduce the exposure to pathogens, especially in areas of high contamination risk during epidemics, such as hospitals or public areas like shops or restaurants.
The device is mounted directly onto the door handle, without causing any damage to it.
In order to assist in the efforts to prevent the spread of COVID-19, Laitek Medical Software will make the design of these devices public and invite the entire community to take and share it, with the suggestion to primarily target hospitals.
Laitek Medical Software grants companies that wish to take over the project for commercial purposes the rights to do so, in accordance with the Terms and Conditions document pertaining to the pack available for download.
Usability
The device can be mounted on door handles with the features specified in the drawing below:
Geometry
Door insert element
Shapes: conical, cylindrical, parallelepipedal
Maximum angle of door insert element: 12 degrees
Maximum thickness of insert element: 27 mm
Minimum edge spacing:
12 mm from the door frame to the edge of the insert element
31 mm from the door to the inner edge of the lever
Lever
Shapes: conical, cylindrical, parallelepipedal, curved, wavy
Height: 35 mm
Thickness: 20 mm
General model features
The device is monolithic, consisting of a single piece that is mounted onto the door handle with three plastic fasteners (zip ties, plastic clips).
There are two versions of the model meant to cover both door opening directions – left / right.
Mounting
To mount the device, you only need three 3,5 mm thick and 30 cm long plastic fasteners (zip ties, plastic clips).
Securing the zip ties once installed, makes the process easier. Notice the wide loop created by the 2nd zip tie.
Note: We recommend securing the zip ties after insertion, at a length of 1 cm from the top. Otherwise, especially when attaching the device onto the door insert element, it will be difficult to insert them once the device is in place.
Insertion of the device onto the door handle
Note: Notice the manner of insertion of the first loop.
Securing the zip ties
Note: Gradually secure each fastening element to ensure that the device is as secure as possible and that it makes maximum contact with the surface of the various types of door handles it is mounted on.
This handle will NOT perfectly fit on any door handle, but this is not an issue. The fastening elements will ensure the necessary rigidity.
3D printing specs
Filament type: PETG / ABS
Required filament: 138 cubic cm for the main part
Layer height: 0,4 mm
Recommended infill: 40-60%
We recommend printing with adhesive on the printing surface.
Temperatures may vary depending on the selected printing speed.
Nozzle temperature: 258 degrees Celsius
Bed temperature: 90 degrees Celsius
Average printing time with the configuration above: 3 h 20 min
Recommended printing position:
Annex – Terms and Conditions
Terms and Conditions for the use and production of the forearm/elbow-operated door opening device.
Definitions
“The Device” – a plastic handle mounted on existing door opening devices (handles)
“The Company” – Laitek Medical software
“Beneficiaries” – the users of the devices designed by the Company
“Manufacturer” – a natural or legal person who produces the device designed by the Company
The Company shall provide the plan of the device to the entire community, with the objective of joining the fight against COVID-19, having no financial claim under the Terms of this document.
Selling the device
Selling the device, by any means, during the state of emergency and the ongoing COVID-19 epidemic period is strictly forbidden.
The moment the epidemic period ends is considered to be the moment when the Romanian Government makes an official announcement in this respect.
Following the end of the epidemic – it becomes mandatory to mention “Designed by Laitek Medical Software” in the documentation and promotion of this commercial product and only with the prior written approval of the Company.
Failure to comply with the provision of letter b) will automatically lead to the Manufacturer’s liability for payment of compensation to the Company.
Device-related information
The Product is not a medical device.
The Product can help limit the spread of the virus but does not eliminate the need for its disinfection or for hands’ sanitization.
Production of the device
The Company produces the device prototypes in a limited number, without committing itself to ensure a sufficient production to meet the demand.
Any natural or legal person can produce the device at their own expense.
Changes to the device are allowed, given a prior notification of the Company.
Device installation
The Company does not undertake to install the devices inside hospitals, the Beneficiary being exclusively responsible for this task.
Device delivery
The delivery of the device shall be done voluntarily. The Company does not assume any responsibility for the delivery of devices created by the community.
Warranties and service
The Company does not provide any type of warranty on the design.
The Company provides free support during the COVID-19 epidemic within the limit of the Laitek volunteers’ available time.
This article was first featured at https://www.thingiverse.com/thing:4355708 on May 13, 2020 at 12:00AM by igoron







More Stories
Can this possibly be true? “Metal 3D printing is now possible on any 3D printer…with the right settings and a few minor upgrades like a hardened steel nozzle…” – July 2 2023 at 04:59PM
New NASA Funding Ignites 25 3D Printing Projects in Space Exploration – June 18 2023 at 04:34PM
Nvidia AI produces 3D models from 2D videos 3D printing applications forthcoming? – June 15 2023 at 02:55AM